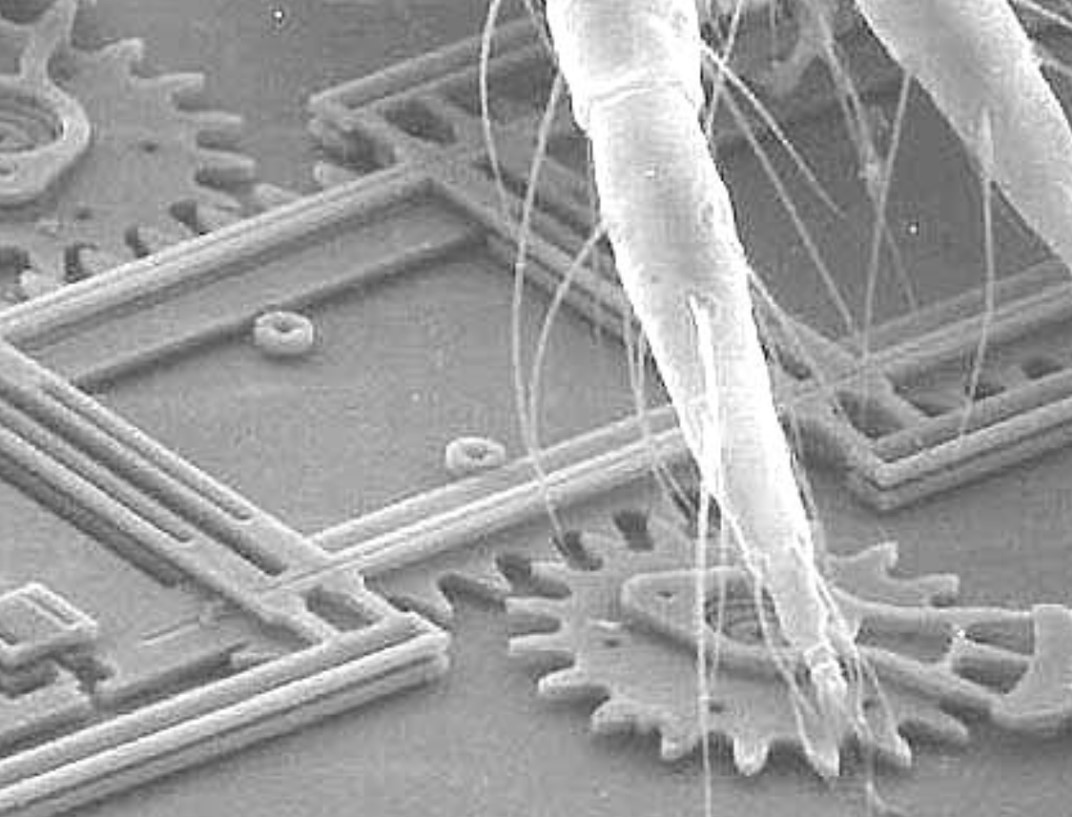
CO2 is a Manufacturing Technology
Cleaning, Cooling, and Lubrication
Carbon dioxide (CO2) is a colorless, odorless, and naturally occurring chemical compound made up of one carbon atom covalently double bonded (resonantly) to two oxygen atoms. Carbon dioxide exists in Earth's atmosphere as a trace gas at a concentration of about 400 ppm. Natural sources include volcanoes, hot springs, and geysers. It is present in deposits of petroleum (liquid), natural gas, and calcium carbonate (limestone). It is released from limestone by heat and pressure (sublimation) and by dissolution in water and acids. Because carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, in ice caps and glaciers, and also in seawater. Major industrial sources of CO2 include fermentation, fertilizer production, energy production, and petroleum oil processing plants. CO2 is continuously generated from and/or transformed into various carbon-based compounds - liquid, gas, and solid - through numerous natural and industrial processes such as photosynthesis, fermentation, combustion, and chemical synthesis. CO2-laden emissions from natural and industrial sources are captured, purified, liquefied, stored, and distributed for reuse in many industrial processes.