Bondability
CO2 Technology Improves Bondability
A manufactured product may require component surfaces to be joined using methods such as gluing, welding, soldering, and coating. The ability of a surface to be cohesively or adhesively joined or bonded to another surface is termed bondability.
Bondability is a measure of the nature and extent of cohesive or adhesive bond strength between joined surfaces. Bonding strength measurements vary according to a particular joining application and include for example bond pull testing, bond lap shear testing, accelerated environmental stress testing, coating peel testing, coating scratch testing, and wire bond pull testing, among several other examples. As with wettability testing, surface tension and surface free energy of the joined surfaces provide a useful metric for optimizing adhesive or cohesive bonding.
Bonding is the act of assembling (or joining) similar or dissimilar materials to build complex structures or devices, or the creation of new beneficial surfaces that address technological needs such as joint strength, abrasion resistance, corrosion resistance, or biocompatibility. Bonding technologies are numerous and include adhesive bonding, mechanical fastening, laser welding, soldering, brazing, acoustic bonding, diffusion bonding, isothermal solidification bonding, transient liquid phase bonding, exothermic bonding, dip coating, and thermal spray coating, among many other examples.
Bonding processes by any method and for any purpose permit the utilization of new engineered materials (i.e., alloys, composites, nanostructured materials, advanced medical devices) in combination with transformative design concepts. In many cases, the methods by which materials are assembled or joined are as important if not more important than the materials used. As such the bonding requirement is critical in the design and manufacturing phase. Example bonding processes comprise:
Adhesive Bonding: liquid adherend (i.e., curable adhesive) joins two or more solid adherends (metal-plastic); liquid adherend cures to form a composite bonded assembly.
Dip Coating: liquid adherend (i.e., coating) applied to a solid adherend (lens); liquid adherend cures to form a coated lens.
Wire Bonding: molten solid adherend (i.e., metallic wire) joined to a solid adherend (bond pad); molten wire cools to form a wire bonded pad.
Welding: molten sold adherend (i.e., metal electrode) joining two molten solid adherend surfaces (metal-metal); molten adherends cool to form a weldment.
Optimal bondability is achieved by optimizing both cleanability and wettability. Optimal wettability may require surface modifications to provide the proper functional chemistry in terms of dispersion, polar, and hydrogen bonding parameters. Beneficial carbon, oxygen, nitrogen, hydroxyl, or silane-based functional groups may be attached to the bonding surfaces to promote the formation of strong adhesive or cohesive bonds. Maximum bondability conditions are achieved with all critical aspects optimized, for example good joint design, adherend chemistry, and cure schedules, etc. Moreover, the principles “like-dissolves-like” and “like-seeks-like” should be applied. The liquid adhesive or coating cohesion parameters and solid surface cohesion energies of adherends should be matched as closely as possible and the chemical reactivity between the adherends is optimized (i.e., formation of strong acid-base pairs).
Given this, optimal surface preparation is the essential first step to provide consistent and reliable adhesive or cohesive bond strength. As described in Figure 2-3 major bonding elements comprise substrate, adhesive, bond line surface area and joint design. Key bonding factors that influence adhesion are represented as vertices on the bonding diagram.
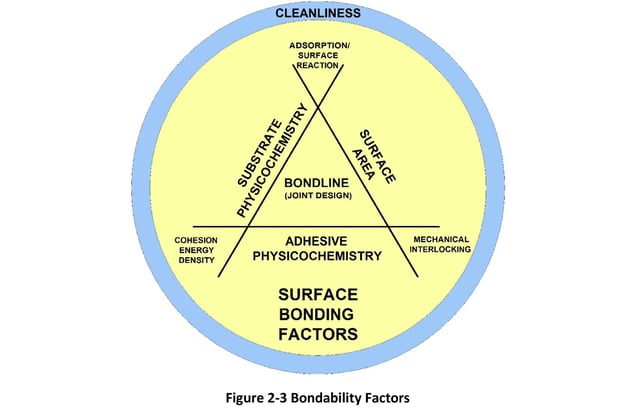
These factors must be optimized as needed for a particular bonding application to obtain maximum adhesion (or cohesion) between the bonding elements. Bonding factors relate to several functional aspects of the bonding interface or bond line and include the following:
1. Maximizing mechanical interlocking: Increasing the microscopic surface roughness (not necessarily surface area) increases “lock and key” physical anchoring between the adhesive and surface. A microscopically rough surface increases the area of physical contact and enhances wettability through capillary flow.2. Matching cohesion energy: The degree of cohesion energy match of the adhesive with the surface should be optimized to create a highly wettable bonding interface. Establishment of such an interface decreases stress in the bond line because continuity exists between the various surfaces - substrate and adhesive.
3. Increasing surface absorption and reactivity: Surfaces should have sites that are polar or contain reactive chemistries with which the adhesive reacts to form, for example, acid-base pairs. Examples of reactive sites include surfaces containing hydroxyl, carbonyl, carboxylic acid and other functional groups which serve as chemical anchors.
With respect to adhesive bonding, functional aspects are largely responsible for the ability of an adhesive to wet a technical surface completely and uniformly. Wettability is a function of the surface energy of both the adhesive and the substrates. This also includes the physicochemical properties of the adhesive such as the viscosity and surface tension, all of which should be matched to the cohesive energy of the substrate or functionalized surfaces.
An important first step prior to (and sometimes following) preparation of bonding surfaces is precision cleaning to achieve a high degree of surface cleanliness. Surface cleanliness is defined as the absence of foreign materials deposited on bonding surfaces. These include fingerprints, particles, manufacturing process residues, vapors, machining oils, loosely adhering oxides, and other surface contaminant layers. Moreover, following surface treatment processes such as microabrasive blasting, surfaces are always populated with microscopic abrasive and ablated particle debris. All these contaminants mask the bonding surface, filling and coating microscopic valleys and asperities with residues that reduce surface area availability which results in reduced bond line adhesion or cohesion strength. Surface contamination also interferes with the functionalization of technical surfaces, masking the surface from the beneficial actions of treatment processes such as for example atmospheric plasma treatment used to increase microscopic surface roughness (i.e., plastics) and activation (i.e., oxygenation).
Surface preparation techniques and goals are different for different types of mating substrates and bonding schemes. The three major categories of bonding or joining processes comprise adhesively joining substrates, the application of coatings to substrates, and the direct bonding (cohesively joining) substrates. Several factors, some similar and some different, form the basis for reliable joining processes across the various bonding schemes and processes. Proper surface preparation forms for the foundation for the bond line with important engineering and design considerations such as proper adhesive selection, preparation and application.
Substrate surfaces may contain one or a combination of hydrocarbon films and particles and non-wettable or low energy surfaces. Many different surface treatment methods have been developed to address these challenges and these are as diverse as the many surface treatment challenges confronted by manufacturing engineers, but not without limitations. Conventional techniques such as mechanical abrasion clean by removing contaminated surface. Microabrasive treatments must be exact to prevent physical damage to the surface (overtreatment) and cause secondary particulate surface contamination which must be removed prior to bonding operations. Wipe cleaning techniques using organic solvents such as acetone and methylethylketone (MEK) are undesirable due to odor, toxicity, flammability, or volatile organic compound (VOC) issues. Solvent wiping tends to smear contamination over the critical bonding surfaces and does not effectively remove thin film contaminants trapped or hidden within microscopic surface topography. Conventional plasma treatments used to etch or functionalize surfaces cannot efficiently address thick or uneven contaminant films, inorganic residues, and particles.
Moreover, microabrasive and liquid treatment agents used to clean and promote surface adhesion are messy. Finally, most conventional treatment agents and methods are not easily adapted to automated manufacturing tools and processes and may not be compatible with cleanroom environments. Given these limitations, it is highly desirable to have a more comprehensive surface treatment process that can address the many surface treatment challenges without introducing secondary contaminations or produce effluents which must be treated. Ideally, such a method should comprise both surface cleaning and surface functionalization to properly prepare a technical surface for bonding.






